Who can participate?
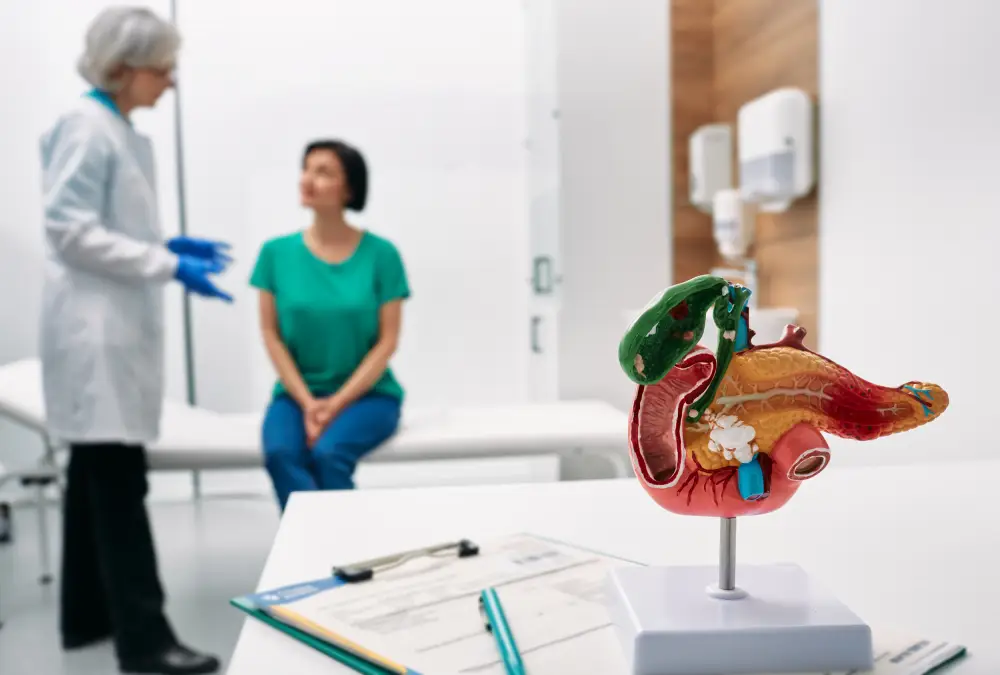
Patients aged 18 years and over with advanced pancreatic ductal adenocarcinoma. In total, the study aims to treat about 120 patients on the trial across the country at several hospitals.
What does the study involve?
The trial will combine VP-002 with two chemotherapies called nab- paclitaxel and gemcitabine, which are both standard treatments given to patients to treat their pancreatic ductal adenocarcinoma. The chemotherapies are given as a drip (infusion) every week, for three in every four weeks. VP- 002 is taken as a tablet twice a day. The study has two stages: in the first stage (Phase I trial), the goal is to work out the best doses of VP- 002 and the chemotherapies together. In the second stage (Phase II trial), the goal is to assess how well this new combination of VP-002 when given chemotherapy works compared to the standard chemotherapies without VP-002.